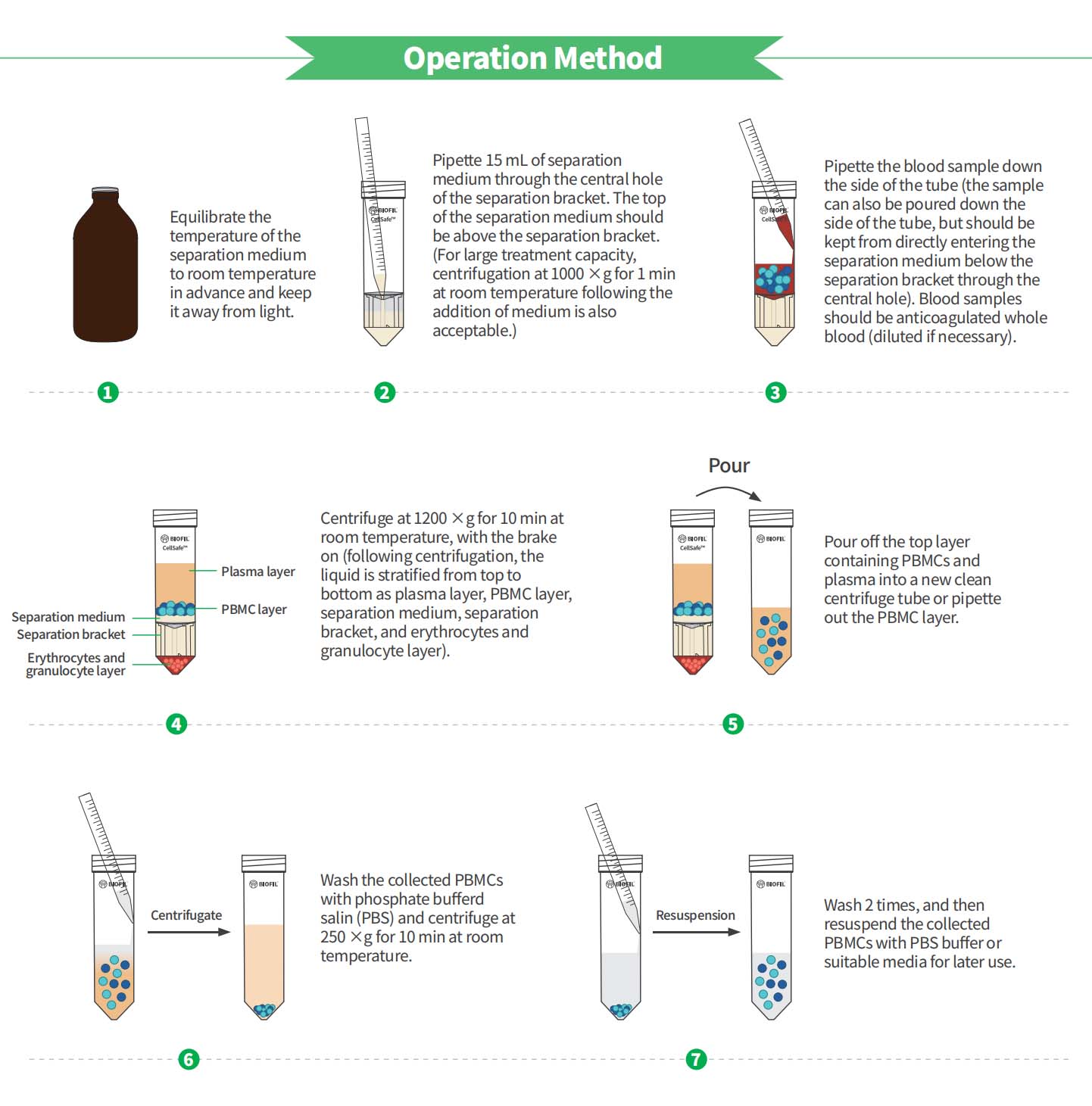
CellSafe™ Peripheral Blood Separation Tube is mainly used to isolate mononuclear cells (MNCs) from peripheral whole blood, cord blood or bone marrow by density gradient centrifugation.
The JET BIOFIL CellSafe™ Peripheral Blood Separation Tube boasts a unique design featuring a built-in separation bracket, which effectively reduces the mixing of target samples and the density gradient medium. This design allows Mononuclear Cells (MNCs) to be retained above the separation bracket, separating them from the erythrocytes and granulocyte layer present at the tube's bottom. MNCs can be collected by effortlessly pouring out after centrifugation without the need for complex steps , streamlining experiments and saving valuable time compared to traditional methods.CellSafe™ Peripheral Blood Separation Tube strictly adheres to the Good Manufacturing Practice (GMP) standards. It meets the requirements for biological laboratory consumables with a higher cleanliness grade, ensuring its suitability for various experimental applications.
Specification: 50 mL with separation bracket Cap Type: Flat
Separation Bracket Type: Eight-hole Cylindrical Bottom Type: Conical

*Tube Body: Polypropylene (PP);
*Tube Cap: High-density polyethylene (HDPE);
*Separation Bracket: Methyl methacrylate-butadiene-styrene (MBS).
*Conforming to USP CLASS VI

*The built-in separation bracket minimizes the mixing of the sample and the separation medium, thereby avoiding the need for slow and laborious application of the sample to the upper layer of the density gradient medium.
*Easy to operate, with MNCs collected by directly pouring out after centrifugation.
*High consistency minimizes the inflfluence of human operation on experimental outcomes.
*Rapidly isolates peripheral blood mononuclear cells (PBMCs) within a 15-minute time frame.
*This product is manufactured in strict adherence to GMP standards, and the fifinished items undergo rigorous third-party testing to meet the experimental requirements for consumables with a higher cleanliness grade.
*Triple independent bagged clean medical outer packaging, with product lot number marked on the
innermost layer for traceability.
*Sterilized by irradiation, SAL 10-6, DNase/RNase-free, Non-pyrogenic, Non-cytotoxic , No mycoplasma.
|
Cat. No. |
Description |
Sterile |
Recommended Sample Volume |
|
|
|
Undiluted |
Diluted |
||||
|
CSP021050 |
Tube with separation bracket (50 mL/tube) |
Y |
4-17ml |
15-30ml |
25/100 |